El grup d’Immunologia Translacional de l’Institut de Recerca Vall d’Hebron (VHIR), liderat pel Dr. Roger Colobran, ha publicat recentment un article on descriu mutacions de pèrdua de funció en heterozigosi al gen TRAF3 com a causa de la immunodeficiència comuna variable (IDCV).
El treball ha estat realitzat en col·laboració amb tot el grup de professionals de l’hospital Vall d’Hebron involucrats en les immunodeficiències primàries. Les primeres autores d’aquest treball són la Blanca Urban (Immunòloga Clínica) i la Dra. Laura Batlle Masó (Investigadora post-doctoral i experta en bioinformàtica).
ABSTRACT:
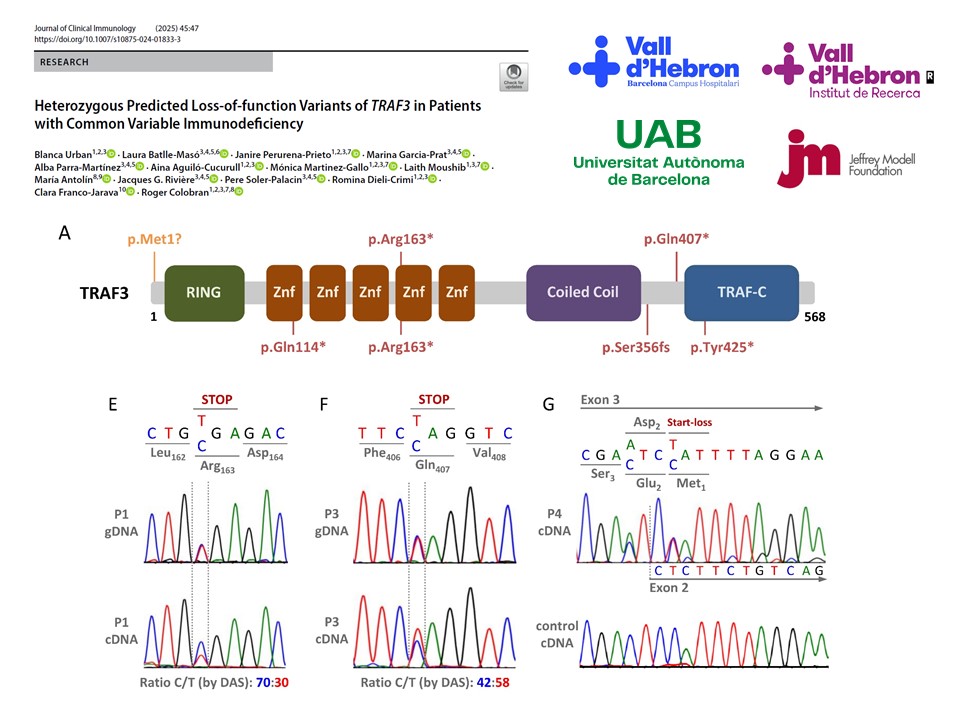
TRAF3, a versatile adaptor protein within the TRAF family, participates in various signaling pathways involving the tumor necrosis factor receptor, toll-like receptor, and retinoic acid-inducible gene I-like receptor families. In 2010, autosomal dominant TRAF3 deficiency was reported in a patient with herpes simplex virus-1 encephalitis, consistent with the role of TRAF3 in type I interferon production. Recently, a novel, completely different clinical phenotype was described in patients with TRAF3 haploinsufficiency (TRAF3Hl), characterized by recurrent bacterial infections, autoimmune features, systemic inflammation, and hypergammaglobulinemia. In this study, we conducted a TRAF3-targeted reanalysis of next-generation sequencing data from 800 patients with inborn errors of immunity. Through this reassessment and additional familial investigations, we identified three previously unidentified cases of TRAF3Hl within two different families. These individuals harbored stop-gain variants (p.Arg163* and p.Gln407*) and experienced recurrent bacterial infections with hypogammaglobulinemia. Previously, the patients had been diagnosed with common variable immunodeficiency (CVID) and were receiving immunoglobulin replacement therapy. In addition, a TRAF3 start-loss variant (c.3G > A) was identified in a fourth patient, but after familial and molecular studies, it was not considered disease-causing, excluding TRAF3Hl in this patient. This study illustrates the usefulness of targeted reanalysis of genes with reported novel phenotypes. We rescued three patients with TRAF3Hl, presenting similarities and differences with the previously reported patients. The most significant differences were hypogammaglobulinemia and a CVID-like presentation. These data expand the clinical phenotype of TRAF3Hl and pave the way for further investigation into loss-of-function variants in patients with CVID.
Enllaç a l’article: